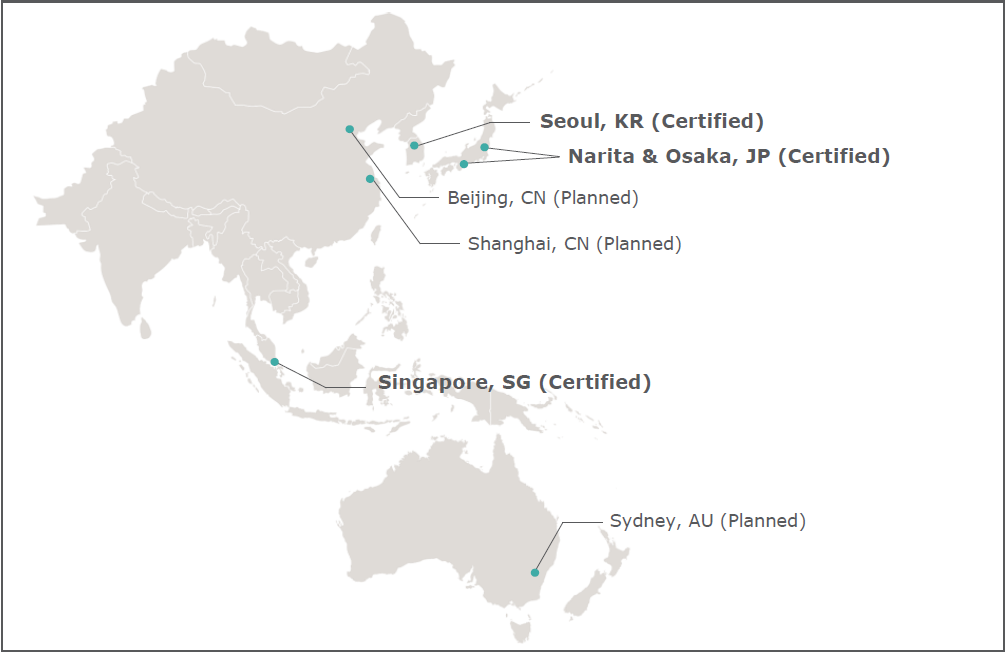
SINGAPORE, February 25, 2022 – UPS (NYSE: UPS) announced today that three of its freight forwarding operations located at Narita International Airport and Kansai International Airport in Japan, and Incheon International Airport in South Korea, have received the prestigious IATA CEIV Pharma Certification[1] for excellence in pharmaceutical air transportation and handling.
Together with its freight forwarding operations at Changi International Airport in Singapore that achieved certification in 2019[2], four of UPS’s freight forwarding operations are now internationally recognized as able to handle and transport high-value pharmaceutical products safely, securely, and efficiently. The certification also validates UPS Healthcare™ Cold Chain Freight service offering at the four locations as being aligned to global best practices.
A globally recognized standard in transporting pharmaceutical air cargo
Without adequate global standardization across the entire air cargo supply chain, critical healthcare cargo such as vaccines and other biopharmaceuticals can be rendered compromised and potentially harmful to patients when subject to even the slightest temperature deviations.
The path towards obtaining certification saw UPS engage in rigorous preparations across multiple aspects of operations, including pharmaceutical product handling and transportation, staff competency, procedures, risk assessment, and infrastructure.
“The latest IATA CEIV Pharma Certifications provides healthcare decision makers, and our pharmaceutical and life sciences customers, with confidence and peace of mind knowing that their products are handled in line with the highest levels of regulatory compliance. This also represents UPS’s commitment to deepening its healthcare capabilities in Asia Pacific in line with global quality standards and compliance,” said Wes Wheeler, President, UPS Healthcare.
Freight forwarding network and expertise underpinning healthcare cold chain logistics
Growth in cold chain logistics spending is expected to accelerate and reach US$21.3 billion by 2024[3]. The uptick in spending underscores increasing demand for specialized healthcare operations and research needs particularly in Asia, where well-established life sciences hubs continue to thrive in Singapore, Japan, and South Korea.
“Through the strong capabilities of our freight forwarding network in Asia coupled with decades of specialized healthcare expertise, UPS is well-equipped to operate a continuous cold chain that is fundamental to protecting product integrity and quality of high-value temperature-sensitive biopharmaceuticals, such as vaccines and clinical trials,” said Anita Li, Vice President, UPS Freight Forwarding, North and South Asia.
“UPS Freight Forwarding in Asia is proud to continue leading the way across our network with these certifications. Looking ahead, UPS will pursue certifications for freight forwarding operations located in Shanghai and Beijing in China, and Sydney in Australia,” added Li.
UPS Healthcare continues to expand capabilities across Asia Pacific
UPS Healthcare recently introduced a comprehensive suite of cold chain technologies, best-in-class capabilities, and new and expanded global facilities to provide end-to-end temperature-controlled logistics. Last year, UPS increased cold chain good manufacturing practices (GMP) storage capacities – roughly 390,000 square feet of coolers – to support the specialized storage of biologics.
UPS Healthcare’s recently launched Marken GMP depot facilities in Japan and South Korea, along with the ongoing expansion of an existing Sydney facility, serve to connect and support the Asia Pacific region with global markets for special storage, compliance and handling needs for pharmaceuticals, biotechnology, and medical devices.
“Patient safety is a shared responsibility of all stakeholders. Our ability to move time-and-temperature-sensitive pharmaceuticals relies on the robustness of our supply chain. As we continue to grow our service offering, we remain committed to enhancing quality excellence that patients around the world continue to depend on for the safe arrival of critical healthcare products,” concluded Wheeler.
As of 2021, UPS delivered more than one billion COVID-19 vaccine doses globally with 99.9% on-time performance, and continues to deliver millions of doses throughout Asia.
[1] IATA CEIV Pharma Certification, International Air Transport Association
[2] UPS enhances Singapore healthcare supply chain capabilities
About UPS
UPS (NYSE: UPS) is one of the world’s largest companies, with 2021 revenue of $97.3 billion, and provides a broad range of integrated logistics solutions for customers in more than 220 countries and territories. Focused on its purpose statement, “Moving our world forward by delivering what matters,” the company’s 534,000 employees embrace a strategy that is simply stated and powerfully executed: Customer First. People Led. Innovation Driven. UPS is committed to reducing its impact on the environment and supporting the communities we serve around the world. UPS also takes an unwavering stance in support of diversity, equality, and inclusion. More information can be found at www.ups.com, about.ups.com and www.investors.ups.com.
About UPS Healthcare
UPS Healthcare delivers unparalleled healthcare logistics expertise to its customers around the world. UPS Healthcare has 11+ million square feet of cGMP and GDP-compliant healthcare distribution space globally. Services include inventory management, cold chain packaging and shipping, storage and fulfillment of medical devices, and lab and clinical trial logistics. UPS Healthcare's global infrastructure, its UPS® Premier visibility service, its track and trace technology, and its global quality system are well-suited to meet today's complex logistics demands for the pharmaceutical, medical device, and laboratory diagnostic industries.